Product Description
Monkeypox Virus Nucleic Acid Detection Kit (PCR-Fluorescence Probing) | IS-FZ-023-25 /IS-FZ-023-50 | BioGerm
Intended Use
This kit is used for qualitative detection of nucleic acid of Monkeypox Virus(MPV )in bloodand Rash exudate from individuals who are suspected of Monkeypox Virus by their healthcare provider. And it is indicated for use as an aid in the diagnosis and monitoring of Monkeypox Virus infection.
Detection Principle
According to the principle of fluorescence PCR technology, this kit designs specific primers and Taqman probes for Monkeypox Virus, and detects the nuclei acid of Monkeypox Virus through fluorescence PR detector.
Main Components
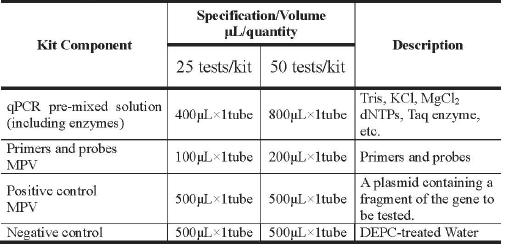
Storage Conditions and Shelf Life
1. Stored at -25°C~-15°C and keep away from light; shelf life: 12 months.
2. Low temperature transportation cannot exceed 4 days; Stored at -25°C - -15°C and keep away from light after opening, no effect on the shelf life, Avoid repeated freezing and thawing. Six times of freezing-thawing will not affect the detection effect.
3. Date of production and shelf life: See the outer packing box
Components Required But Not Included within the Test
1. Alternative extraction reagents: Nucleic acid extraction and purification kit (Shanghai BioGerm Medical Technology Co., Ltd.)
2. Consumables not supplied: 1.5 ml. DNase-free and RNase-free Eppendorf tube 0.2 mL PCR tube or strip Various models of pipettes and pipette tips (10uL, 200uL and 1000uL. tips with filters) Centrifuge (can reach to 12,000 rpm) Microcentrituge Disposable powder-free gloves and surgical gowns Etc.
3. Real-Time PR Instrument(s); CFX96 Dx System (Bio-Rad Inc.), Applied Biosystems 7500 Real-Time PCR Instrument System (Thermo Fisher Scientific Inc.). LightCycler8 480 Real-Time PR System (Roche Molecular Systems, Inc.) and so on.
Requirements for Sample
1. Types of samples: Blood (collect fresh anticoagulant blood) Rash exudate.
2. Sample collection: all specimens should be collected by a medical professional at a designated place following collection instructions of the collection device manufacturer.
3. Storage conditions: Collected specimens should be submitted for inspection in time, Specimens tested within 24 hours should be stored at 4°C, and stored at -70°C for more than 24 hours, and repeated freeze-thaw should be avoided.
Detection Method:
1. Reagent preparation (reagent preparation area) Thaw the kit components at 4°C and keep away from light, shake and mix thoroughly, and then centrifuge immediately. Calculate the number of reagents used N (N = number of samples 1 < positive control > + 1 < negative control >), prepare the reaction system according to the following table, add it to a centrifuge tube of an appropriate volume, shake and mix well then centrifuge immediately, aliquot 20 ul, into PR reaction tubes respectively and transfer to the sample processing area.

2. Sample processing (sample processing area)
- Nucleic acid extraction: Select the appropriate nuclei acid extraction kit to extract nuclei acid of virus, and operate according to the instructions of the corresponding kit.
- Loading: Add 5 uL of the extraction of sample, 5 uL of positive control and 5 uL of negative control to the PCR reaction tubes prepared above, and ensure that the final volume is 25 uL/tube. Tightly cap the tube or sealing film, and centrifuge immediately, and then place the PCR reaction tubes in a fluorescence quantitative thermal cycler for amplification detection.
3. PCR amplification detection (amplification detection area)
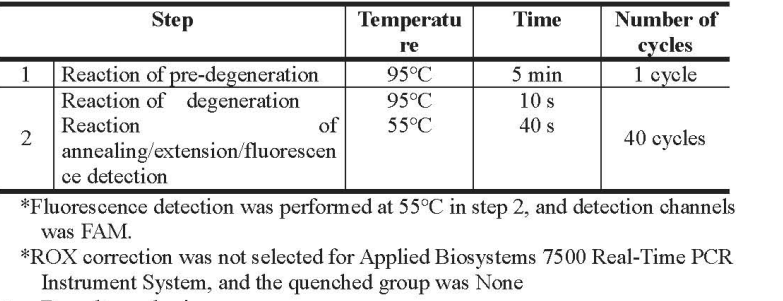
4. Result analysis
Adjust the start and end values of baseline as well as threshold value according to the image after analysis (it is recommended to set the start value at 3-15 and the end value at 5-20, and adjust the amplification curve of negative control to be straight or lower than the threshold line), click Analysis to automatically obtain the analysis results, and view the results on the Report interface.
Quality control: Each test must include the Positive control and Negative control result. Acceptance Criteria of Controls:
1. Negative control(valid) : Ct value > 38 or no detection.
2. Positive control(valid) : the amplification curve was S-shaped, and the Ct value was ≤30.
If the negative and/or positive control included in the run is invalid, the entire run is invalid and patient results cannot be interpreted. In this case a root cause analysis needs to be performed, and all patient specimens need to be retested after the root cause has been identified and eliminated.
Interpretation of the results:
All test controls should be examined prior to interpretation of patient results. If the controls are not valid, the patient results cannot be interpreted.
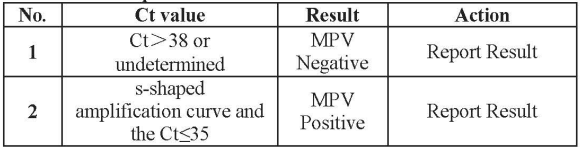

Note:
1. If a suspicious sample re-test, and the retest result is s-shapedamplification curve and 35 <Ct≤38,the result is positive.
2. FAM channel was used to detect MPV.
Limitations of the Detection Method:
1. Improper sample collection, transportation and storage, and improper reagent transportation, storage and configuration may affect the detection results, or even could lead to false negative results.
2. False positive results may occur it laboratory contamination, reagent contamination, and sample cross-contamination occur
Performance characteristics:
1. Limit of detection: 5×10° copies/mL
2. Specificity: MPV can be detected in all specimens and there is no overlap with other types.
Warnings and Precautions:
 Euro
Euro
 USD
USD
 British Pound
British Pound
 NULL
NULL








