Product Description
FARS2 Antibody | 29-387 | ProSci
Host: Rabbit
Reactivity: Human
Homology: N/A
Immunogen: Antibody produced in rabbits immunized with a synthetic peptide corresponding a region of human FARS2.
Research Area: Other
Tested Application: E, WB, IHC
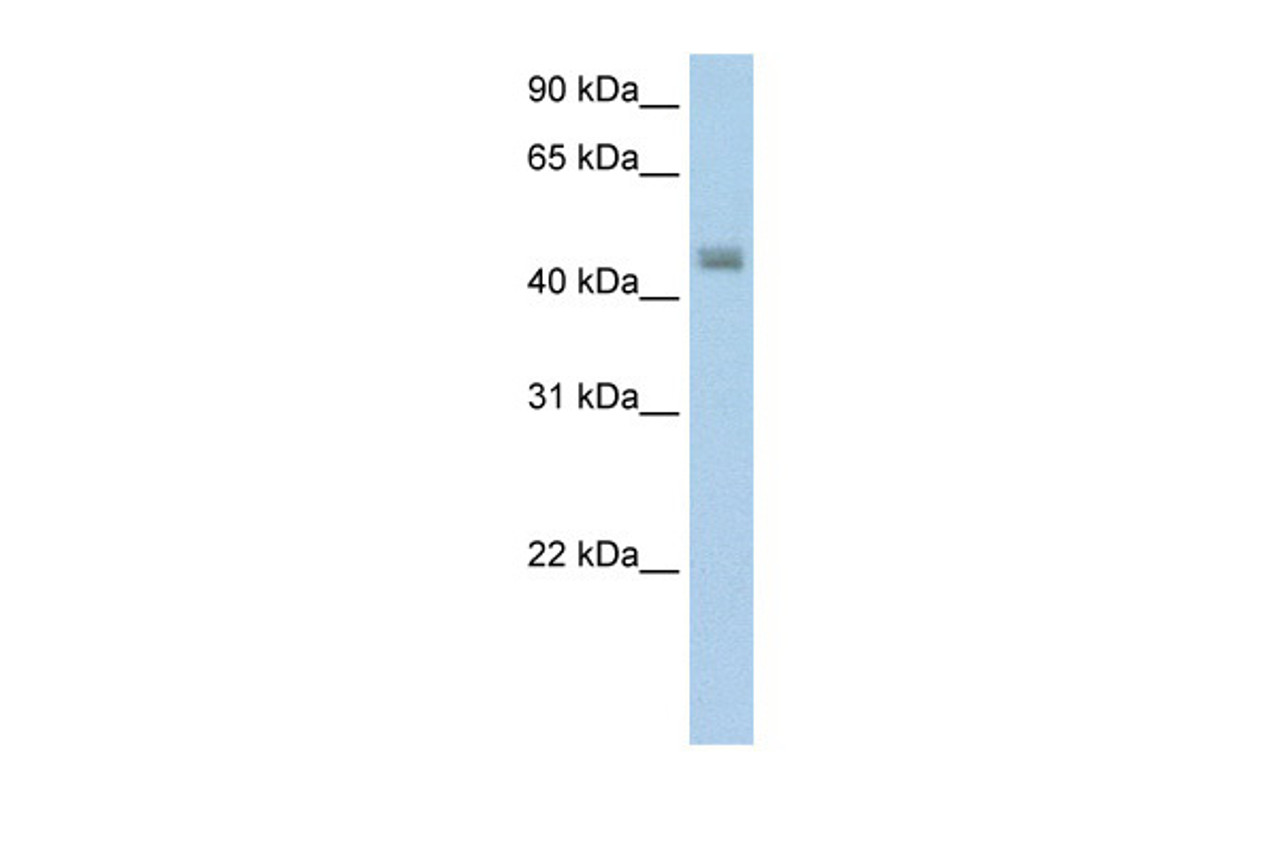
Application: FARS2 antibody can be used for detection of FARS2 by ELISA at 1:62500. FARS2 antibody can be used for detection of FARS2 by western blot at 5.0 μg/mL, and HRP conjugated secondary antibody should be diluted 1:50, 000 - 100, 000.
Specificiy: N/A
Positive Control 1: Tranfected 293T Cell Lysate
Positive Control 2: N/A
Positive Control 3: N/A
Positive Control 4: N/A
Positive Control 5: N/A
Positive Control 6: N/A
Molecular Weight: 50 kDa
Validation: N/A
Isoform: N/A
Purification: Antibody is purified by protein A chromatography method.
Clonality: Polyclonal
Clone: N/A
Isotype: N/A
Conjugate: Unconjugated
Physical State: Liquid
Buffer: Purified antibody supplied in 1x PBS buffer with 0.09% (w/v) sodium azide and 2% sucrose.
Concentration: batch dependent
Storage Condition: For short periods of storage (days) store at 4˚C. For longer periods of storage, store FARS2 antibody at -20˚C. As with any antibody avoid repeat freeze-thaw cycles.
Alternate Name: FARS2, FARS1, HSPC320, PheRS, dJ520B18.2, COXPD14
User Note: Optimal dilutions for each application to be determined by the researcher.
BACKGROUND: Aminoacyl-tRNA synthetases are a class of enzymes that charge tRNAs with their cognate amino acids. FARS2 is a phenylalanine-tRNA synthetase (PheRS) localized to the mitochondrion which consists of a single polypeptide chain, unlike the (alpha-beta) 2 structure of the prokaryotic and eukaryotic cytoplasmic forms of PheRS. Structure analysis and catalytic properties indicate mitochondrial PheRSs may constitute a class of PheRS distinct from the enzymes found in prokaryotes and in the eukaryotic cytoplasm.Aminoacyl-tRNA synthetases are a class of enzymes that charge tRNAs with their cognate amino acids. FARS1 encodes a mitochondrially-located phenylalanine-tRNA synthetase (PheRS) which consists of a single polypeptide chain unlike the (alpha-beta) 2 structure of the prokaryotic and eukaryotic cytoplasmic forms of PheRS. Structure analysis and catalytic properties indicate mitochondrial PheRSs may constitute a class of PheRS distinct from the enzymes found in prokaryotes and the eukaryotic cytoplasm.
 Euro
Euro
 USD
USD
 British Pound
British Pound
 NULL
NULL