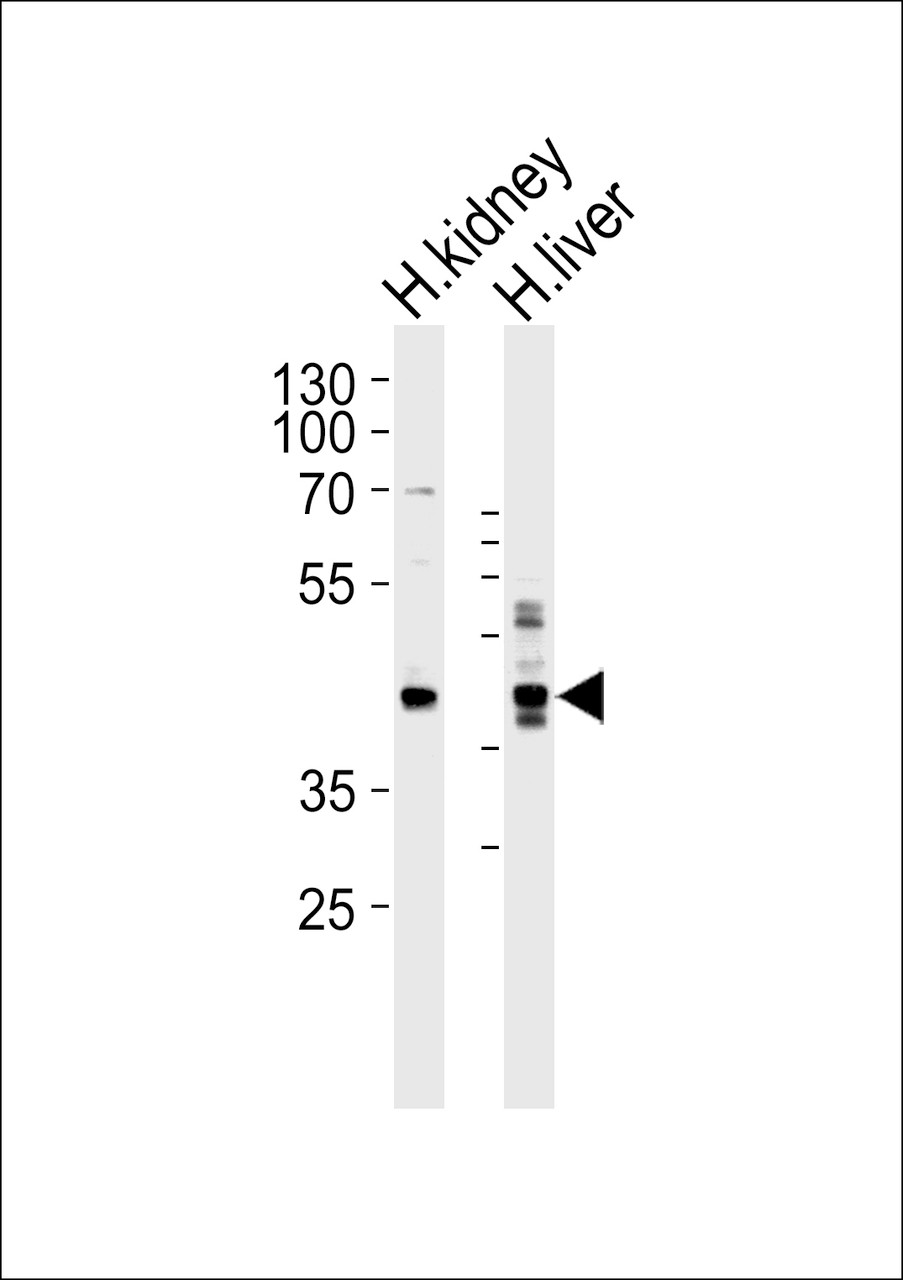
Product Description
SEPT9 Antibody | 62-182 | ProSci
Host: Rabbit
Reactivity: Human
Homology: N/A
Immunogen: This SEPT9 antibody is generated from rabbits immunized with a KLH conjugated synthetic peptide between 57-85 amino acids from the C-terminal region of human SEPT9.
Research Area: Cell Cycle
Tested Application: WB, IHC-P, Flow
Application: For WB starting dilution is: 1:1000
For IHC-P starting dilution is: 1:50~100
For FACS starting dilution is: 1:10~50
Specificiy: N/A
Positive Control 1: N/A
Positive Control 2: N/A
Positive Control 3: N/A
Positive Control 4: N/A
Positive Control 5: N/A
Positive Control 6: N/A
Molecular Weight: 65 kDa
Validation: N/A
Isoform: N/A
Purification: This antibody is purified through a protein A column, followed by peptide affinity purification.
Clonality: Polyclonal
Clone: N/A
Isotype: Rabbit Ig
Conjugate: Unconjugated
Physical State: Liquid
Buffer: Supplied in PBS with 0.09% (W/V) sodium azide.
Concentration: batch dependent
Storage Condition: Store at 4˚C for three months and -20˚C, stable for up to one year. As with all antibodies care should be taken to avoid repeated freeze thaw cycles. Antibodies should not be exposed to prolonged high temperatures.
Alternate Name: Septin-9, MLL septin-like fusion protein MSF-A, MLL septin-like fusion protein, Ovarian/Breast septin, Ov/Br septin, Septin D1, SEPT9, KIAA0991, MSF
User Note: Optimal dilutions for each application to be determined by the researcher.
BACKGROUND: The maf oncogene was identified by structural analysis of the AS42 avian transforming retrovirus genome. The Maf family is divided into two subclasses, large Mafs (vMaf, cMaf, MafB and Nrl) and small Mafs (MafF, MafK, and MafG) . Both subclasses contain leucinezipper motifs, which allow homodimerization as well as heterodimerization with a variety of other bZip transcription factors. Large Mafs also contain an acidic transactivation domain absent in the small Maf proteins. Although they do not possess inherent transactivation activity, small Maf proteins can act as positive regulators of transcription by targeting transcriptionally active dimerization partners to specific DNA regulatory elements. Conversely, small Mafs can act also as negative regulators of transcription by recruiting transcriptional repressors or by forming homodimers that can replace active dimers. Human MafF was isolated in a yeast one-hybrid system from a human myometrium cDNA library. Human MAFF encodes a 164 amino acids proten. Like other small MAFF proteins, it contains an extended leucine zipper structure and lacks an N-terminal transactivating domain. The three small Maf proteins have been implicated in a number of physiological processes, including development, differentiation, haematopoiesis and stress response. Interestingly, these three proteins regulate the stress response via different mechanisms.
 Euro
Euro
 USD
USD
 British Pound
British Pound
 NULL
NULL