Product Description
Baricitinib | 11-074 | ProSci
BNR: Chemical
Predicted Molecular Weight: 371, 4
Physical State: Lyophilized
Storage Condition: Short Term Storage: +4C. Long Term Storage: -20C. Handling Advice: Keep cool and dry. Use/Stability: Stable for at least 2 years after receipt when stored at -20C.
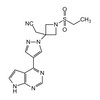
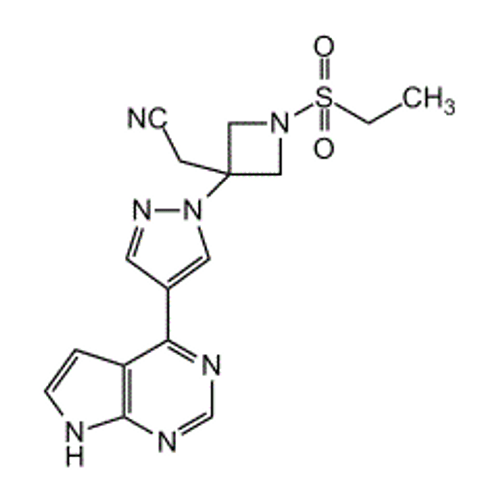
Alternate Name: LY3009104; INCB28050; 1- (Ethylsulfonyl) -3-[4- (7H-pyrrolo[2, 3-d]pyrimidin-4-yl) -1H-pyrazol-1-yl]-3-azetidineacetonitrile
Background: Baricitinib is a selective orally bioavailable JAK1/JAK2 inhibitor with nanomolar potency against JAK1 (IC50 = 5.9nM) and JAK2 (IC50 = 5.7nM) and inhibits Tyk2 (IC50 = 53nM) . It displays >100-fold selectivity for JAK1/2 over JAK3 (IC50 > 400nM) . Janus kinases (JAKs) are non-receptor kinases and play important roles in the proinflammatory signaling pathways that are frequently over-activated in autoimmune disorders such as rheumatoid arthritis. Upon binding of extracellular cytokines and growth factors, JAKs are phosphorylated and activate signal transducers and activators of transcription (STATs) . Via these signaling cascades, inflammatory cytokine and chemokine transcription is induced to form inflammatory mediators including IL-2, IL-6, IL-12, IL-15, IL-23. Baricitinib inhibits intracellular signaling of multiple proinflammatory cytokines including IL-6 and IL-23 at concentrations <50nM. It inhibits IL-6 receptor signaling, IL-6-induced STAT phosphorylation and subsequent pro-inflammatory chemokine (MPC-1) and cytokine (IL-17 and IL-22) production in PBMCs and T cells and displays anti-inflammatory and disease modifying effects in the rat adjuvant arthritis model. It also has been shown to block MSU-induced inflammasome activation. Baricitinib has potential application in various inflammatory disorders, including rheumatoid arthritis and systemic lupus erythematosus (SLE) . The majority of viruses enter cells through receptor mediated endocytosis. Baricitinib potently inhibits AP-2 associated protein kinase 1 (AAK1) and also binds cyclin G-associated kinase, both regulators of endocytosis. Inhibiting AAK1 might interrupt the passage of the SARS-CoV-2 virus into cells and also the intracellular assembly of virus particles. In addition the drug’s anti-inflammatory activity is expected to act on the inflammatory cascade associated with COVID-19.
Disclaimer: This product is for research use only.
Purity: greater than or equal to 98% (HPLC)
Formula: C16H17N7O2S
Source: N/A
Solubility: Soluble in DMSO (30mg/ml) or DMF (40mg/ml) .
InChi Key: XUZMWHLSFXCVMG-UHFFFAOYSA-N
Smiles: CCS (N (C1) CC1 (CC#N) N (N=C2) C=C2C3=NC=NC4=C3C=CN4) (=O) =O
RTECS: N/A
Identity: Determined by 1H-NMR.
Merck Index: N/A
 Euro
Euro
 USD
USD
 British Pound
British Pound
 NULL
NULL