Product Description
Niclosamide . ethanolamine | 10-034 | ProSci
BNR: Chemical
Predicted Molecular Weight: 327.1 . 61.1
Physical State: Lyophilized
Storage Condition: Short Term Storage: +4C. Long Term Storage: -20C. Handling Advice: Keep cool and dry.Protect from moisture and oxygen Use/Stability:Stable for at least 2 years after receipt when stored at -20C.
Alternate Name: Niclosamide-olamine; Clonitralide; Bayluscide; BAY-6076; HL2448; Mollutox; 2', 5-Dichloro-4'-nitro-salicylanilide 2-aminoethanol salt
Background: With slightly better solubility properties compared to Niclosamide (Prod. No. AG-CR1-3643) . Antihelminthic, molluscicide and trypanocidal agent that inhibits mitochondrial oxidative phosphorylation and anaerobic ATP production. Anticancer agent with antiproliferative activity in a broad spectrum of cancer cells. Targets and inhibits multiple signaling pathways, including STAT3, NF-kB, Wnt/β-catenin, Notch and mTORC1. Shown to block TNF-α-induced IκBα phosphorylation, translocation of p65 and the expression of NF-κB-regulated genes and elevates reactive oxygen species (ROS) levels. Promotes Frizzled1 internalization, down-regulates the expression of Dishevelled-2 protein and inhibits β-catenin stabilization. Inhibits mTORC1 but not mTORC2 signaling in cells maintained in nutrient-rich conditions. Cell permeable selective STAT3 inhibitor (IC50=0.25μM) . Inhibits the activation, nuclear translocation and transactivation of STAT3. Selective over STAT1, STAT5, JAK1, JAK2 and Src kinases. Induces cell cycle arrest, growth inhibition, autophagy and apoptosis. Inhibits S100A4 (Metastasin-1) -induced metastasis in vivo. Inhibits androgen receptor (AR) splice variants. Anti-inflammatory compound. Quorum sensing inhibitor. Broad-spectrum antiviral agent that targets acidified endosomes. Has been shown to inhibit replication of SARS and is a potential candidate to treat SARS-CoV-2 (COVID-19) infections. Antiobesity and antidiabetic agent. Positive allosteric neuropetide Y4 receptor ligand and increases energy expenditure and lipid metabolism through mitochondrial uncoupling.
Disclaimer: This product is for research use only.
Purity: greater than or equal to 98%
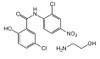
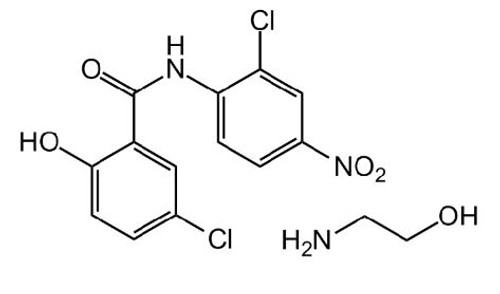
Formula: C13H8Cl2N2O4 . C2H7NO
Source: N/A
Solubility: Soluble in DMSO (20mg/ml) .
InChi Key: XYCDHXSQODHSLG-UHFFFAOYSA-N
Smiles: OC1=CC=C (Cl) C=C1C (NC2=CC=C ([N+] ([O-]) =O) C=C2Cl) =O.NCCO
RTECS: VN8575000
Identity: N/A
Merck Index: 5, 110416667
 Euro
Euro
 USD
USD
 British Pound
British Pound
 NULL
NULL