Product Description
RNH1 Antibody | 56-428 | ProSci
Host: Rabbit
Reactivity: Human
Homology: N/A
Immunogen: This RNH1 antibody is generated from rabbits immunized with a KLH conjugated synthetic peptide between 425-454 amino acids from the C-terminal region of human RNH1.
Research Area: Other
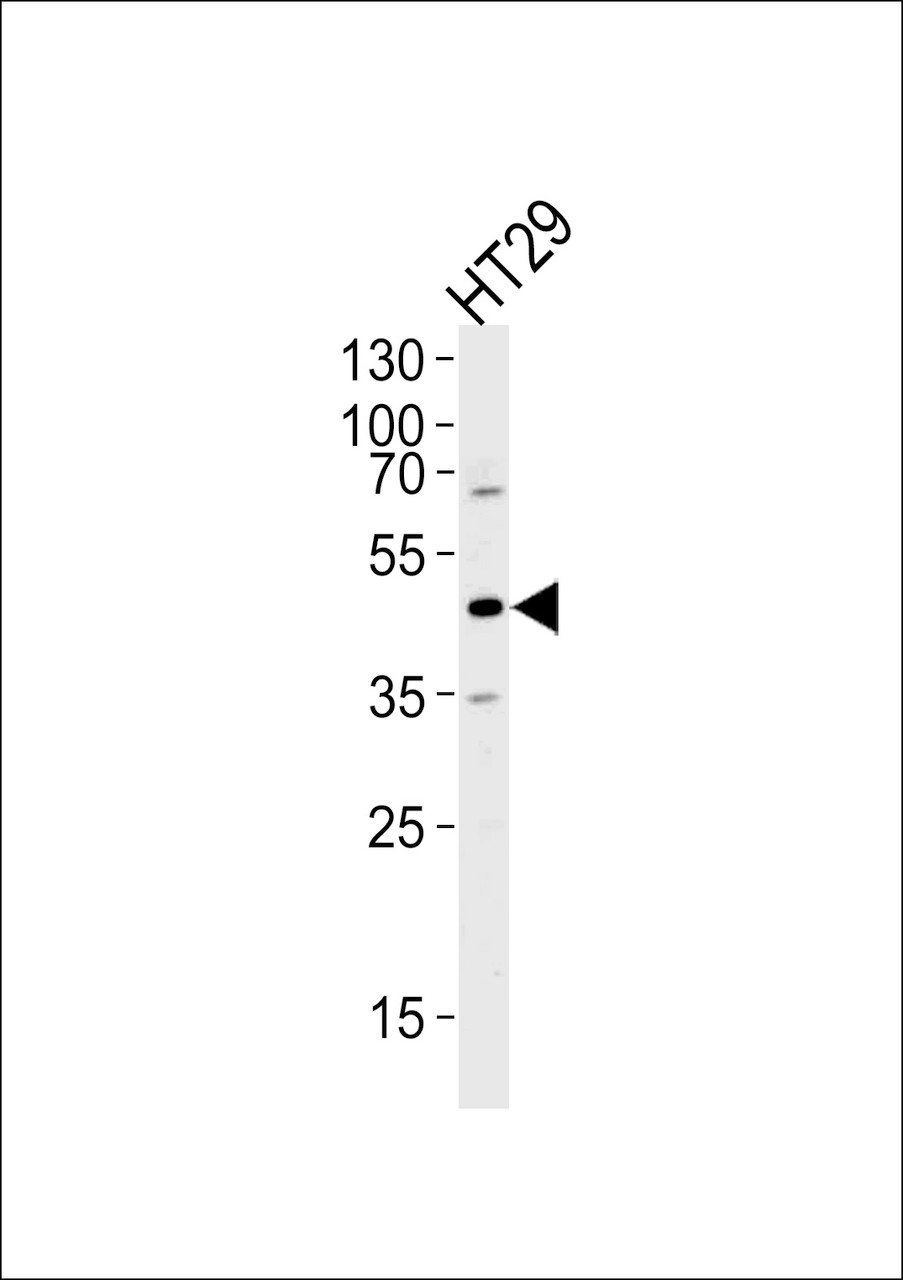
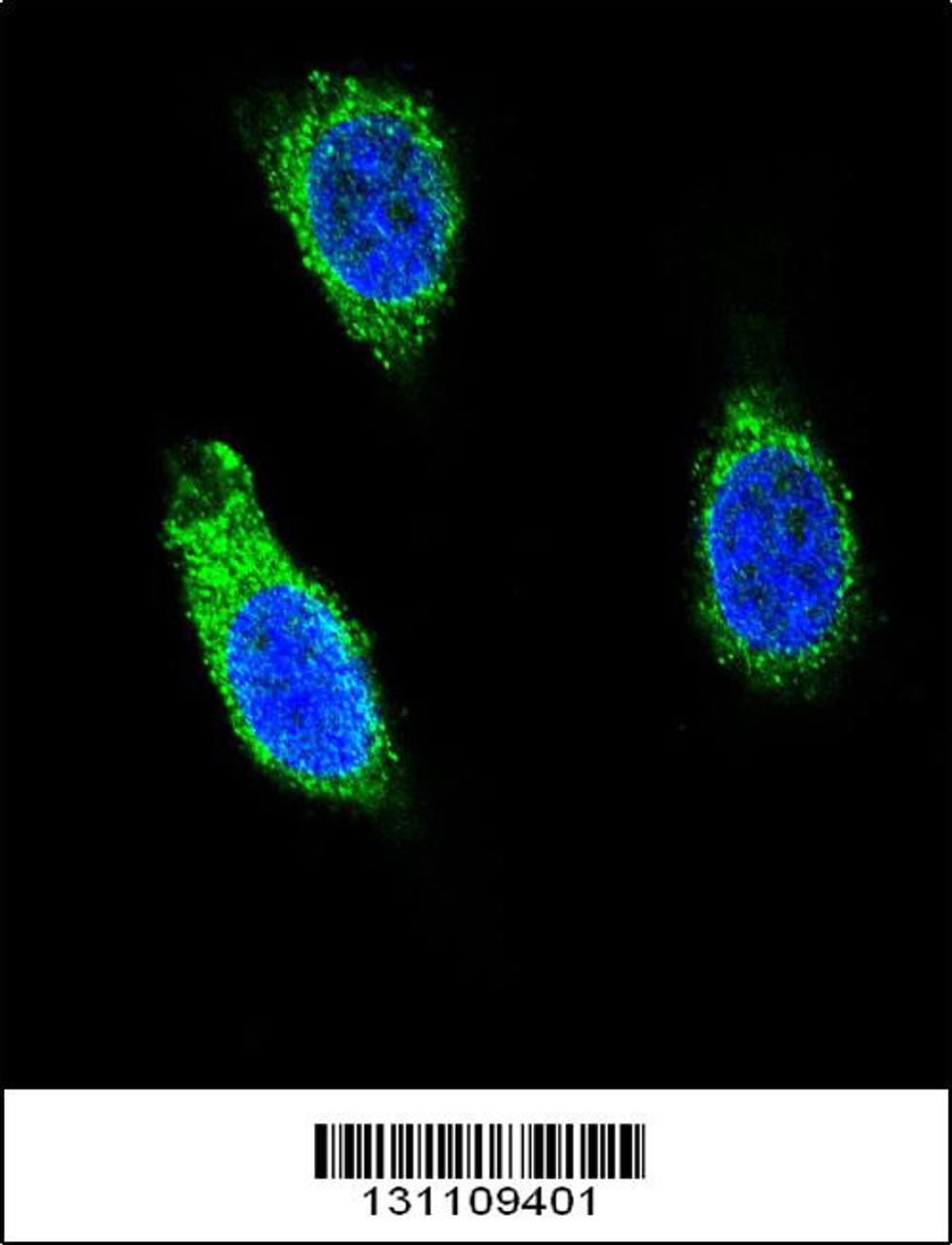
Tested Application: WB, IF
Application: For WB starting dilution is: 1:1000
For IF starting dilution is: 1:10~50
Specificiy: N/A
Positive Control 1: N/A
Positive Control 2: N/A
Positive Control 3: N/A
Positive Control 4: N/A
Positive Control 5: N/A
Positive Control 6: N/A
Molecular Weight: 50 kDa
Validation: N/A
Isoform: N/A
Purification: This antibody is purified through a protein A column, followed by peptide affinity purification.
Clonality: Polyclonal
Clone: N/A
Isotype: Rabbit Ig
Conjugate: Unconjugated
Physical State: Liquid
Buffer: Supplied in PBS with 0.09% (W/V) sodium azide.
Concentration: batch dependent
Storage Condition: Store at 4˚C for three months and -20˚C, stable for up to one year. As with all antibodies care should be taken to avoid repeated freeze thaw cycles. Antibodies should not be exposed to prolonged high temperatures.
Alternate Name: Ribonuclease inhibitor, Placental ribonuclease inhibitor, Placental RNase inhibitor, Ribonuclease/angiogenin inhibitor 1, RAI, RNH1, PRI, RNH
User Note: Optimal dilutions for each application to be determined by the researcher.
BACKGROUND: Placental ribonuclease inhibitor (PRI) is a member of a family of proteinaceous cytoplasmic RNase inhibitors that occur in many tissues and bind to both intracellular and extracellular RNases (summarized by Lee et al., 1988 [PubMed 3219362]) . In addition to control of intracellular RNases, the inhibitor may have a role in the regulation of angiogenin (MIM 105850) . Ribonuclease inhibitor, of 50, 000 Da, binds to ribonucleases and holds them in a latent form. Since neutral and alkaline ribonucleases probably play a critical role in the turnover of RNA in eukaryotic cells, RNH may be essential for control of mRNA turnover; the interaction of eukaryotic cells with ribonuclease may be reversible in vivo.
 Euro
Euro
 USD
USD
 British Pound
British Pound
 NULL
NULL