Product Description
Androgen Receptor (phospho Tyr363) Antibody | 79-872 | ProSci
Host: Rabbit
Reactivity: Human, Mouse
Homology: N/A
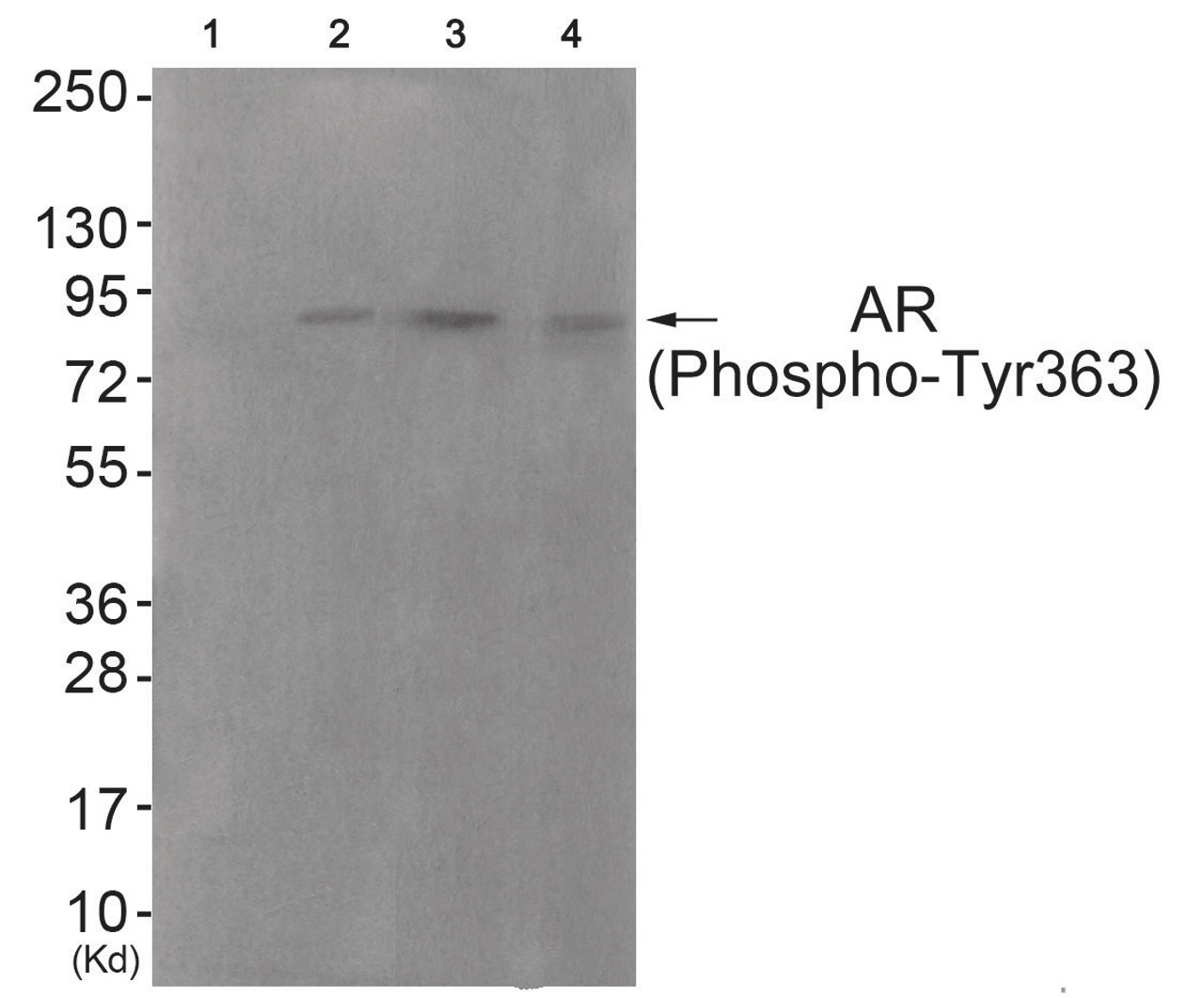
Immunogen: Androgen Receptor (phospho Tyr363) antibody was raised against a peptide sequence around phosphorylation site of tyrosine 363 (D-Y-Y (p) -N-F) derived from Human Androgen Receptor.
Research Area: Phospho-Specific
Tested Application: WB
Application: Western Blot: 1:500~1:1000
Specificiy: The antibody detects endogenous levels of Androgen Receptor only when phosphorylated at tyrosine 363.
Positive Control 1: N/A
Positive Control 2: N/A
Positive Control 3: N/A
Positive Control 4: N/A
Positive Control 5: N/A
Positive Control 6: N/A
Molecular Weight: 85 kDa
Validation: N/A
Isoform: N/A
Purification: Antibodies were purified by affinity-chromatography using epitope-specific peptide.
Clonality: Polyclonal
Clone: N/A
Isotype: IgG
Conjugate: Unconjugated
Physical State: Liquid
Buffer: Antibody supplied in phosphate buffered saline (without Mg2+ and Ca2+) , pH 7.4, 150mM NaCl, 0.02% sodium azide and 50% glycerol.
Concentration: 1 mg/mL
Storage Condition: Store antibody at -20˚C for up to one year.
Alternate Name: ANDR, Androgen receptor, Dihydrotestosterone receptor, NR3C4
User Note: N/A
BACKGROUND: The androgen receptor gene is more than 90 kb long and codes for a protein that has 3 major functional domains: the N-terminal domain, DNA-binding domain, and androgen-binding domain. The protein functions as a steroid-hormone activated transcription factor. Upon binding the hormone ligand, the receptor dissociates from accessory proteins, translocates into the nucleus, dimerizes, and then stimulates transcription of androgen responsive genes. This gene contains 2 polymorphic trinucleotide repeat segments that encode polyglutamine and polyglycine tracts in the N-terminal transactivation domain of its protein. Expansion of the polyglutamine tract causes spinal bulbar muscular atrophy (Kennedy disease) . Mutations in this gene are also associated with complete androgen insensitivity (CAIS) . Two alternatively spliced variants encoding distinct isoforms have been described.
 Euro
Euro
 USD
USD
 British Pound
British Pound
 NULL
NULL