Product Description
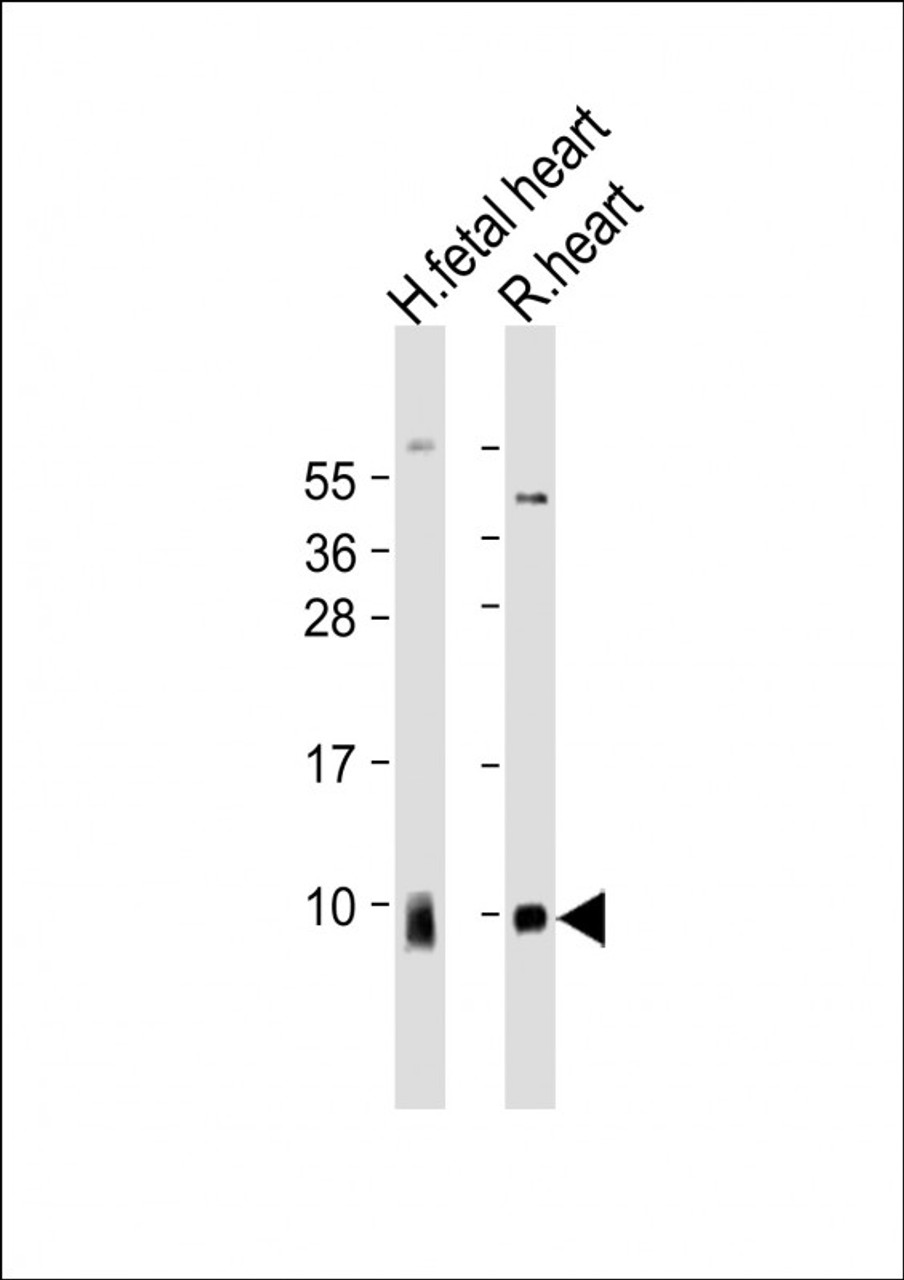
ATP5G1 Antibody | 61-317 | ProSci
Host: Rabbit
Reactivity: Human, Rat
Homology: Predicted species reactivity based on immunogen sequence: Bovine, Mouse, Pig, Sheep
Immunogen: This ATP5G1 antibody is generated from a rabbit immunized with a KLH conjugated synthetic peptide between 41-71 amino acids from the Central region of human ATP5G1.
Research Area: Obesity, Signal Transduction
Tested Application: WB
Application: For WB starting dilution is: 1:2000
Specificiy: N/A
Positive Control 1: N/A
Positive Control 2: N/A
Positive Control 3: N/A
Positive Control 4: N/A
Positive Control 5: N/A
Positive Control 6: N/A
Molecular Weight: 14 kDa
Validation: N/A
Isoform: N/A
Purification: This antibody is purified through a protein A column, followed by peptide affinity purification.
Clonality: polyclonal
Clone: N/A
Isotype: Rabbit Ig
Conjugate: Unconjugated
Physical State: Liquid
Buffer: Supplied in PBS with 0.09% (W/V) sodium azide.
Concentration: batch dependent
Storage Condition: Store at 4˚C for three months and -20˚C, stable for up to one year. As with all antibodies care should be taken to avoid repeated freeze thaw cycles. Antibodies should not be exposed to prolonged high temperatures.
Alternate Name: ATP synthase F (0) complex subunit C1, mitochondrial, ATP synthase lipid-binding protein, ATP synthase proteolipid P1, ATP synthase proton-transporting mitochondrial F (0) complex subunit C1, ATPase protein 9, ATPase subunit c, ATP5G1
User Note: Optimal dilutions for each application to be determined by the researcher.
BACKGROUND: Mitochondrial membrane ATP synthase (F (1) F (0) ATP synthase or Complex V) produces ATP from ADP in the presence of a proton gradient across the membrane which is generated by electron transport complexes of the respiratory chain. F-type ATPases consist of two structural domains, F (1) - containing the extramembraneous catalytic core and F (0) - containing the membrane proton channel, linked together by a central stalk and a peripheral stalk. During catalysis, ATP synthesis in the catalytic domain of F (1) is coupled via a rotary mechanism of the central stalk subunits to proton translocation. Part of the complex F (0) domain. A homomeric c-ring of probably 10 subunits is part of the complex rotary element.
 Euro
Euro
 USD
USD
 British Pound
British Pound
 NULL
NULL









![ATP5G1 Antibody (Center) [APG02124G] ATP5G1 Antibody (Center) [APG02124G]](https://cdn11.bigcommerce.com/s-452hpg8iuh/images/stencil/500x659/products/865501/1157568/logo__92149.1659788186__31910.1659862491.png?c=2)


![ATP5G1 Antibody (Center) [APG02123G] ATP5G1 Antibody (Center) [APG02123G]](https://cdn11.bigcommerce.com/s-452hpg8iuh/images/stencil/500x659/products/870312/1162379/logo__92149.1659788186__84569.1659866489.png?c=2)
