Product Description
Monkeypox Virus Real Time PCR Test Kit | N2301 | Beta-Bear Laboratory
Intended Use
The Monkeypox Virus Real Time PCR Test Kit is a real-time (rt) polymerase chain reaction (PCR) test intended for the qualitative detection of nucleic acid from monkeypox in skin lesion exudate swabs, lesion roofs and lesion crusts material collected at a healthcare location or collected by a healthcare provider, from individuals suspected of monkeypox infection by their healthcare provider. Testing is limited to professional use. Results are for the identification of Monkeypox virus DNA. The Monkeypox virus DNA is generally detectable in skin lesion specimens during the acute phase of infection. Positive results are indicative of the presence of Monkeypox virus DNA; clinical correlation with patient history and other diagnostic information is necessary to determine patient infection status. Positive results do not rule out bacterial infection or co infection with other viruses. Negative results do not preclude Monkeypox virus infection and should not be used as the sole basis for patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information. The NOVA Test® Monkeypox Virus Real Time PCR Test Kit is intended for use by qualified and trained clinical laboratory personnel specifically instructed and trained in the techniques of real-time PR and in vitro diagnostic procedures. For in vitro diagnostic use only.
Method
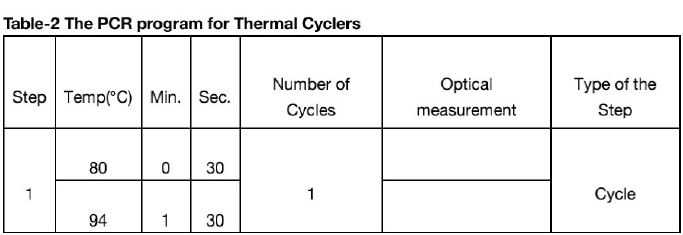
The NOVA Test® Monkeypox Virus Real Time PCR Test Kit is based on fluorescent modification of the PCR method. The PCR-mix contains target-specific probes bearing reporter fluorescent dyes and quencher molecules. Once hybridized to a target sequence, the probes become activated. As a result of activation fluorescence increases proportionally to target sequence amplification. The intensity of fluorescence is measured at every cycle of reaction with a Real- time PR thermal cycler data collection unit and analyzed with the software provided. The PCR-mix includes the Internal control (IC), which is intended to assess the quality of the polymerase chain reaction. DNA probes used for the detection of the Monkeypox Virus product amplification include fluorescent dyes Fam and Cy5. DNA probe used for the detection of the internal control amplification product includes the fluorescent dye Hex.

Reagents
1. Monkeypox Virus Internal Control (4 vials, 1.2mL per vial)
2. PCR Amplification Butfer (96 tests) Include synthetic oligonucleotides(primers and probes), dNTPs in a buffered solution with a reference dye. Preservative: 0.10% ProClin 300 and 0.15% ProClin 950.
3. Taq-polymerase solution(1 vial, 0.15mL per vial) 3.0 Units/uL in buffered solution
4. Monkeypox Virus Positive Control (8 vials, 1.5mL per vial)
5. Monkeypox Virus Negative Control (8 vials, 1.5mL per vial)
All components are ready to use and do not require additional preparation for operation. The NOVA Test® Monkeypox Virus Real Time PCR Test Kit is intended for single use and designed for 96 tests.

 Euro
Euro
 USD
USD
 British Pound
British Pound
 NULL
NULL